Churg-Strauss syndrome: A case report
Case presentation
Authors
Abstract
Churg-Strauss Syndrome, currently referred to as Eosinophilic Granulomatosis with Polyangiitis, is a multisystemic disease characterized by asthma and eosinophilia. Diagnostic criteria include Asthma, Abnormalities in the paranasal sinuses, Eosinophilia > 10%, Neuropathy (mono or poly), Pulmonary infiltrates, and Biopsy with vessel involvement containing extravascular eosinophilia. EGPA, which can be confused with many other diseases, should always be considered in the differential diagnosis in the presence of asthma and eosinophilia. In this case, we discussed Churg-Strauss disease diagnosed in a 43-year-old male patient.
Keywords
Introduction
Churg-Strauss Syndrome, also known as Eosinophilic Granulomatosis with Polyangiitis, first described in 1951, is a syndrome characterized by asthma, eosinophilia, eosinophilic infiltration in tissues, and vasculitis, with an unknown etiology. In this case, a 43-year-old male patient, who had been diagnosed with allergic bronchopulmonary aspergillosis (ABPA) for a year, presented with an increase in shortness of breath and complaints of skin lesions while under steroid treatment. As the patient did not respond to steroid and antifungal therapy during follow- ups, a skin biopsy was performed. Eosinophil-rich vasculitic changes were observed in the skin biopsy, consistent with CSS. The patient, diagnosed with CSS based on clinical, laboratory, and pathological findings, was started on cyclophosphamide treatment. We aimed to highlight the importance of differential diagnosis in the ongoing follow- up of the patient.
Case Presentation
A forty-three-year-old male patient, under follow-up for asthma for the past two years, presented with increased shortness of breath, dark sputum, and hemoptysis for the last month. The patient had been diagnosed with nasal polyposis for four years and underwent nasal polypectomy surgery two years ago. He has a history of smoking four packs a year, and coarse rales were noted during the physical examination. Other systemic examinations were unremarkable. Due to the presence of a non-regressing consolidated area on the chest X-ray, a chest CT was performed (Figure 1). The chest CT revealed dilated bronchi filled with high-density mucus in a central distribution throughout all lobes of both lungs. The patient was admitted to our service with a preliminary diagnosis of allergic bronchopulmonary aspergillosis (ABPA). The admission white blood cell count was 19,000 with neutrophils at 53% and eosinophils at 31%. The total IgE was 1229, and the sed rate was 59. The patient’s skin prick test for Aspergillus was negative, as were Aspergillus-specific IgE and galactomannan tests. Bronchoscopy was performed, revealing widespread dark secretions in both lungs. The cytology of bronchoalveolar lavage was eosinophil-rich. Radiologically and clinically, the patient was diagnosed with ABPA, and treatment with prednisolone and itraconazole was initiated.
The patient, who has been under observation for approximately one year, presented to us with newly developed skin lesions due to the lack of regression in symptoms during follow-up. Lesions were observed on the distal tibia in front of the ankle, characterized by scattered, intense, pink-red petechial-style spots, and the formation of plaques by their combination (Figure 2). Mild coarse rales were present upon auscultation of the respiratory system. Other system examinations were normal. Blood pressure: 130/70, pulse: 105, respiratory rate: 20, saturation: 91. Total IgE was 3990, and peripheral blood eosinophils were 63% (1800). ANA, RF, and other collagen markers, as well as p-ANCA and c-ANCA, were negative. Parasitological examination of the stool yielded negative results. Echocardiography and abdominal ultrasound were evaluated as normal. Sinus CT revealed widespread mucosal thickening and opacifications related to secretion in the paranasal sinuses (Figure 3). A skin biopsy showed eosinophil-rich vasculitic changes, consistent with Churg-Strauss syndrome (CSS). The patient, diagnosed with CSS based on clinical, laboratory, and pathological findings, was started on cyclophosphamide treatment and is currently under our follow-up.
Discussion
Churg-Strauss syndrome (CSS) was first described by Churg and Strauss in 1951. While it can occur at any age, it most commonly manifests between the ages of 38 and 50 (1). CSS tends to exhibit three stages: the first stage characterized by non-specific symptoms of asthma and allergic rhinitis, the second stage featuring hypereosinophilia along with eosinophilic pneumonia or gastroenteritis, and the third stage marked by the onset of systemic vasculitis. These three stages may not always occur sequentially and can sometimes coexist (2, 3).
The criteria set by the American College of Rheumatology in 1990 for the diagnosis of Churg-Strauss syndrome include:
• Asthma
• Abnormalities in the paranasal sinuses
• Eosinophilia > 10%
• Neuropathy (mono or poly)
• Pulmonary infiltrates
• Biopsy with vessel involvement containing extravascular eosinophilia (4).
When four of these criteria are met, a diagnosis can be made with 85% sensitivity and 99.7% specificity.
Etiology of the disease has been implicated in cocaine use, bronchopulmonary aspergillosis, macrolide antibiotics, leukotriene inhibitors such as zafirlukast, and repeated or widespread non-specific immunological stimulation (5). Systemic or inhaled antigens can initiate immunological reactions leading to systemic vasculitis (5). Cases of CSS emerging when steroids are reduced or discontinued in steroid- dependent asthmatic patients have also been reported (6). In our case, while systemic corticosteroid treatment continued due to ABPA, the onset of symptoms related to CSS led us to consider the syndrome as Aspergillus-related.
Skin manifestations, observed in approximately 70% of patients, include erythematous maculopapules resembling erythema multiforme, hemorrhagic lesions ranging from petechiae with accompanying urticaria to widespread ecchymoses, and cutaneous/subcutaneous nodules (2, 6, 7). Purpura and nodules are seen in about two-thirds of patients and are more related to the involvement of small vessels (10). The most common clinical cutaneous lesion is a papule or nodule with a histological structure showing cutaneous extravascular necrotizing granulomas, with a diameter ranging from 2 mm to 2 cm or more (2). Our patient had nodular lesions, especially below 1 cm distal to the tibia, forming a plaque-like appearance. Although not a criterion for the diagnosis of CSS, the association of ANCA, especially anti-MPO, in 70% of patients during the active phase has been identified (2). c-ANCA is common, especially in patients with granuloma formation (2). However, it is not considered a reliable indicator for monitoring disease activity (5). Both c-ANCA and MPO-ANCA were negative in our case. The histopathology of the disease typically shows leukocytoclastic vasculitis in hemorrhagic areas of the skin. Eosinophils may also be prominent. While the granulomatous reaction, initially thought to be a characteristic histopathological feature of CSS, may not always be present, it is not necessary for the diagnosis (3). The histopathological examination of our case revealed eosinophil-rich leukocytoclastic vasculitis, and no granulomatous structure was encountered.
In most cases, asthma precedes vasculitis, and there is a considerable interval between the two (5). Although asthma may improve with the development of vasculitis, it often re-emerges as a significant clinical problem in the post-vasculitis period (2). In our case, there were no active symptoms or findings related to asthma at the time of presentation, but there was a history of asthma. The lung is the most commonly affected organ in CSS, and pulmonary infiltration seen in approximately 90% of patients is one of the 6 ACR criteria used in the diagnosis of CSS (5, 9). Usually, transient, patchy or widespread parenchymal changes or nodules have been reported (9). High- resolution computed tomography of the chest in our case revealed widespread, small micronodular parenchymal lesions. Due to the lack of large randomized, controlled trials comparing different treatment methods for the disease, determining the optimal treatment is challenging (10). While corticosteroids provide dramatic improvement, in cases with severe multisystem involvement, cyclophosphamide and daily prednisone treatment are recommended (10). In our case, five of the ACR criteria were present, and the gold standard, biopsy, was applied. The etiology is not fully explained, but it is considered secondary to Aspergillus, as the patient’s symptoms were highlighted due to the decrease in steroid treatment. Corticosteroids are used in treatment and, in cases of insufficient response or the presence of serious side effects related to corticosteroids, drugs such as cyclophosphamide, methotrexate, or azathioprine may be added. We initiated cyclophosphamide treatment in our case, and follow-ups are ongoing.
Figures
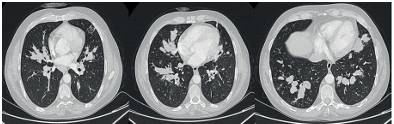
Figure 1. Dilated bronchi filled with high-density mucus in a central distribution throughout all lobes of both lungs.
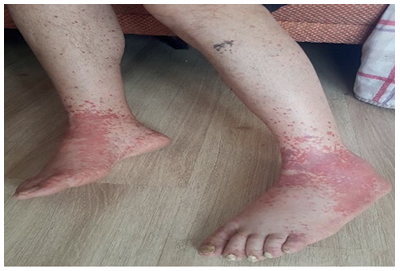
Figure 2. Lesions were observed on the distal tibia in front of the ankle, characterized by scattered, intense, pink-red petechial-style spots, and the formation of plaques by their combination
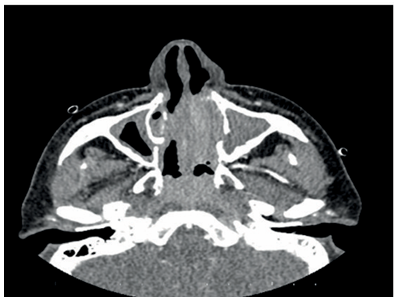
Figure 3. Widespread mucosal thickening and opacifications related to secretion in the paranasal sinuses
Conclusion
In summary, CSS can mimic various other diseases. In this case, we demonstrated that our patient, who had been previously followed up with a different diagnosis, was diagnosed with CSS during subsequent follow-ups.
Data Availability
The data supporting the findings of this article are available from the corresponding author upon reasonable request, due to privacy and ethical restrictions. The corresponding author has committed to share the de-identified data with qualified researchers after confirmation of the necessary ethical or institutional approvals. Requests for data access should be directed to bmp.eqco@gmail.com
References
-
Allen JN, Davis WB. Eosinophilic lung diseases. Am J Respir Crit Care Med. 1994;150:1423-38.
-
Gross WL. Systemic necrotizing vasculitis. Baillieres Clin Rheumatol. 1997;11(2):259-284.
-
Barnhill RL, Busam KJ. Vascular disease. Lever’s histopathology of the skin . Ed. Elder D. Eight edition. Philedelphia New York, Lippincott-Raven Publishers, 1997;185-208.
-
Masi AT, Hunder GG, Lie JT. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum. 1990;33:1094-100.
-
Guillevin L, Cohen P, Gayraud M, Lhote F, Jarrousse B, Casassus P. Churg-Strauss syndrome. Clinical study and long term follow-up of 96 patients. Medicine (Baltimore). 1999;78(1): 26-37.
-
Churg A, Brallas M, Cronin SR, Churg J. Formes frustes of Churg-Strauss syndrome. Chest. 1995;108(2):320-323.
-
Davis MD, Daoud MS, McEvoy MT, Su WP. Cutaneous manifestations of Churg-Strauss syndrome: A clinicopathologic correlation. J Am Acad Dermatol. 1997;37:199-203.
-
Guillevin L, Lhote F, Gherardi R. Polyarteritis nodosa, microscopic polyarteritis, and Churg- Strauss syndrome: Clinical aspects, neurologic manifestations, and treatment. Neurol Clin. 1997;15(4):865-886.
-
Worthy SA, Müller NL, Hansell DM, Flower CD. Churg-Strauss syndrome: The spectrum of pulmonary CT findings in 17 patients. Am J Roentgenol. 1998;170(2):297-300.
-
Fishman JA. The eosinophilic pneumonia. Fishman’s Pulmonary Disease and Disorders. 1998;1133-1150.
Scientific Responsibility Statement
The authors declare that they are responsible for the article’s scientific content including study design, data collection, analysis and interpretation, writing, some of the main line, or all of the preparation and scientific review of the contents and approval of the final version of the article.
Animal and Human Rights Statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflict of Interest
None of the authors received any type of financial support that could be considered potential conflict of interest regarding the manuscript or its submission.
Additional Information
Publisher’s Note
Bayrakol MP remains neutral with regard to jurisdictional and institutional claims.
Rights and Permissions
About This Article
How to Cite This Article
Elif Açar, Orhan Kayakıran, Suat Konuk, Emine Özsarı. Churg-Strauss syndrome: A case report. Eu Clin Anal Med 2024;12(2):33-35
Publication History
- Received:
- December 26, 2023
- Accepted:
- April 22, 2024
- Published Online:
- April 27, 2024
- Printed:
- May 1, 2024
