Plasma pentraxin-3 and growth differentiation factor-15 response to acute aerobic exercise in professional road cyclists
Plasma pentraxın-3 ınprofessıonal roadcyclists
Authors
Abstract
Aim This study aimed to evaluate the concentrations of plasma pentraxin-3 (PTX-3), growth differentiation factor-15 (GDF-15), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) before and after national and international competitions, which represent physically and psychologically intense exercises for elite and young male cyclists, who are endurance athletes.
Material and Methods Eight young male and eight elite male cyclists participated in the study, conducted during two national and international races. The first race covered 116.4 km, and the second covered 122.5 km. Blood samples were collected before the competition, immediately after the race, and 1 hour and 3 hours post-race. The levels of PTX-3, GDF-15, IL-6, and TNF-α in the samples were analyzed using the ELISA method.
Results The PTX-3 level in elite athletes was lower 3 hours after the competition compared to 1 hour after the race (p<0.05). GDF-15 levels in elite athletes were higher 1 and 3 hours after the race compared to before and immediately after the competition but decreased 3 hours after the competition (p<0.05). In young athletes, GDF-15 levels were higher immediately after, as well as 1 and 3 hours post-race, compared to pre-competition levels (p<0.05). IL-6 levels in both young and elite athletes were higher immediately after and 1 hour post-race, compared to pre-competition levels (p<0.05). No significant changes were observed in TNF-α levels (p>0.05).In conclusion, in professional road cyclists, significant changes in GDF-15 and IL-6 levels were observed in response to competition, while PTX-3 and TNF-α levels remained unchanged.
Discussion To determine the causes and sources of the change in these markers, more detailed molecular-level studies are needed, especially with experimental animals.
Keywords
Introduction
The pentraxin superfamily is divided into two subfamilies: short and long pentraxins. C-reactive protein (CRP) and serum amyloid P (SAP) form the short pentraxin branch of the family. The normal plasma concentration of PTX-3 is 0.25–3 ng/ml. CRP and SAP indicate the systemic response to local inflammation by being produced and released in hepatic hepatocytes, while PTX-3 indicates the inflammatory state of the vascular structure as it is released directly from the damaged tissue. PTX-3 expression increases during inflammation, and circulating levels of PTX-3 rise in various pathological conditions affecting the cardiovascular system, including vasculitis, acute myocardial infarction (MI), rheumatoid arthritis, and systemic inflammatory response syndrome/septic shock. Serum levels begin to rise immediately after the onset of inflammation, reaching a maximum value within approximately 7.5 hours, and return to normal within 3–5 days. PTX-3 levels in the blood can increase dramatically from less than two ng/ml to 200–800 ng/ml during inflammatory conditions and active disease. This increase occurs much faster than the rise in CRP levels, making PTX-3 a useful early marker of inflammation.
PTX3 plays a role in the progression of cardiovascular diseases (CVD) through mechanisms such as exacerbating vascular endothelial dysfunction, affecting angiogenesis, and regulating inflammation and oxidative stress. For this reason, PTX-3 can be used as an early marker of inflammation [1]. Clinically, PTX3 shows a positive correlation with arterial hypertension, current-mediated dilatation, and intima- media thickness. Therefore, the role of PTX3 in the pathogenesis and evaluation of endothelial dysfunction is obvious, and it may become a biomarker in this direction [2].
Growth differentiation factor-15 (GDF-15) belongs to the transforming growth factor-beta (TGF-β) superfamily and acts as a hormone or a stress-sensitive circulating factor. GDF-15 is a protein with a molecular weight of 25 kDa. Its physiological concentration in humans is approximately 450 pg/ml. Circulating concentrations of GDF-15 have been shown to increase in various pathological conditions, such as metabolic diseases, inflammation, heart failure, and cardiac hypertrophy. GDF-15 production increases in response to various stimuli, including oxidized low-density lipoprotein (ox-LDL) cytokines such as IL-1β, TNF-α, angiotensin II, TGF-β, and M-CSF. GDF-15 appears to act as an anti-inflammatory factor by inhibiting macrophage activation. Its expression is induced by mitochondrial dysfunction following an increase in reactive oxygen species (ROS), which accelerates aging by damaging essential macromolecules such as DNA, proteins, and lipids. Higher plasma GDF-15 concentrations have been reported in individuals with Down syndrome, who exhibit accelerated aging compared to healthy controls of similar age. As a circulating myokine, GDF-15 increases in response to muscle-specific mitochondrial stress and muscle dysfunction in humans and various mouse models. Increased GDF-15 production appears to allow muscle regeneration and remodeling. Following mitochondrial stress, increased GDF-15 expression in skeletal muscle regulates systemic metabolic homeostasis, increases insulin resistance, and affects lipolysis and oxidative metabolism in the liver, muscle, and white adipose tissues, thereby protecting against diet- induced obesity. GDF-15 plays a protective role similar to neuroglia in the central nervous system (CNS), supporting the survival of dopaminergic neurons and spinal cord motor neurons. GDF-15 is also expressed in adipose tissue and secreted by adipocytes. GDF-15 levels positively correlate with adiponectin levels and negatively correlate with body mass index (BMI) and body fat mass. Obese individuals have increased plasma GDF-15 concentrations, with the highest GDF-15 levels observed in patients with type 2 diabetes.
General Characteristics of Bicycle Tour Races
Tour races are divided into three main branches: mountain bike, road bike, and track (against time) bike. Physiological changes that occur in tour races began to be measured scientifically in the early 1990s with the use of heart rate monitors. The most common and frequently used method is the measurements made during each stage and run of different intensities during the race. Usually, identical phases are created with the heart rates (HR) detected during the previously performed ergometric tests. Measurements made under laboratory conditions or during the race have shown that HR and power output (W) are related, and it has been shown that the value of W can be calculated from HR measurements. With this method, the physiological load (TRIMP) produced during the race is calculated and recorded.
Pentraxin-3 and Exercise
Acute aerobic exercise increases systemic PTX-3 concentrations. Plasma PTX-3 concentrations have been reported to increase for up to 1 hour in response to a single aerobic exercise session. Resting PTX-3 concentrations increase in aerobically active people compared to sedentary men, and cardiorespiratory fitness levels (VO2max) predict acute exercise-induced PTX-3 response following submaximal and maximal levels of aerobic exercise. Plasma PTX-3 concentrations increased after 8 weeks of habitual moderate aerobic exercise training in elderly men and women, and these increases were associated with increased VO2max and improved cardiovascular function indices. It has been shown that the capacity of acute exercise to increase plasma PTX-3 concentrations is similar in normal-weight individuals and obese individuals. This suggests that a single aerobic exercise session is equally beneficial for obese and normal-weight individuals. Also, in response to 12 weeks of aerobic exercise training, high resting PTX-3 concentrations have been associated with a decrease in BMI in obese and overweight women. From these results, it may be thought that increased PTX-3 concentrations may also mediate the anti- inflammatory effects of aerobic exercise in obese individuals.
Growth Differentiation Factor-15 and Exercise
The role of GDF-15 in regulating energy balance has started to attract attention with the demonstration that GDF-15 suppresses food intake. Pharmacological and genetic studies have confirmed that GDF-15 administration reduces body weight largely by reducing appetite. Some studies have also suggested that GDF-15 can directly increase thermogenesis and improve insulin sensitivity. GDF-15 is expressed in many tissues, including skeletal November muscle, and its release is usually increased in response to cellular stress or injury. GDF-15 expression in mouse skeletal November muscle is markedly increased in response to mitochondrial stress. Circulating GDF-15 levels are elevated in patients with muscle atrophy and mitochondrial myopathy. These November Tues indicate that GDF-15 may be released into the circulation from skeletal muscle in response to a stress stimulus.
The Effect of Exercise on IL-6 and TNF-α Levels
Initially, IL-6 was described as a pro-inflammatory cytokine. Subsequently, the anti-inflammatory properties of IL-6 have also been identified.IL-6 is secreted from monocytes, macrophages, fibroblasts, and vascular endothelial cells. When released from macrophages, it shows a pro-inflammatory effect by following the NF-кB signaling pathway. When IL-6 is released from skeletal muscle, it exerts anti- inflammatory action by following the Ca/NFAT and MAPK pathways. An increase in IL-6 levels due to exercise has a suppressive effect on inflammation. An increase in the level of IL-6 due to the secretion of macrophages has an effect that triggers systemic inflammation.
Materials and Methods
Participant Selection
The study was conducted with two groups of cyclists from the TorkuŞekerspor Cycling Team. The first group consisted of elite male cyclists aged 20–36, and the second group consisted of young male cyclists aged 17–18.
Before the study began, athletes were informed about the study, and they signed an informed consent form. All participants had a body mass index (BMI) below 25. None of the participants had any acute or chronic illnesses, and none had smoking or alcohol consumption habits. Additionally, no participants were using anti-inflammatory or steroid-like medications.
For inclusion in the study, athletes in the first group were required to have participated in a training program for at least two years, with three training sessions per week lasting an average of 2 hours each day at an intensity of 15–16 on the Borg scale. For athletes in the second group, the same criteria applied, but for a minimum of one year. Participants who did not meet these training requirements were excluded from the study.
This study was conducted during two different races.
1. The first race was the 116.4 km International Mevlana Cycling Tour, a stage race in Konya. Blood samples were collected from the eight elite male cyclists (Group 1) before the competition, immediately after the race, and 1 and 3 hours post-race.
2. The second race was the 122.5 km National Individual Classification Konya Stage, organized by the Turkish Cycling Federation. Blood samples were collected from the eight young male cyclists (Group 2) before the competition, immediately after the race, and 1 and 3 hours post-race.
Anthropometric Measurements
Before the study began, anthropometric measurements of the athletes were taken. Height was measured in centimeters (cm) using a stadiometer while the athletes stood in an anatomical position, wearing sports clothing but no shoes. Body weight was measured in kilograms (kg) using a digital scale. The body mass index (BMI) was calculated using the formula BMI = weight (kg) / height (m²).
Laboratory Analyses
Analysis of PTX-3 Levels
Plasma PTX-3 levels were measured using a commercially available ELISA kit (Catalog No: MBS772804, MyBioSource ELISA Kits, USA), following the protocol provided with the kit. This kit was a ready-to- use solid-phase enzyme-linked immunosorbent assay (ELISA) kit that worked based on the sandwich principle. PTX-3 levels were expressed as ng/ml. For this kit, the intra-assay coefficient of variation (CV) was less than 10%, the inter-assay CV was less than 15%, and the minimum detection limit was 0.1 ng/ml.
Analysis of GDF-15 Levels
Plasma GDF-15 levels were analyzed using an Invitrogen ELISA kit (Catalog No: BMS2258, Vienna, Austria). Analyses were performed according to the instructions provided with the kit. GDF-15 levels were expressed as pg/ml. For this kit, the intra-assay CV was 4.2%, the inter- assay CV was 2.9%, and the minimum detection limit was 0.589 pg/ml.
Analysis of IL-6 Levels
Plasma IL-6 levels were analyzed using an Invitrogen ELISA kit (Catalog No: BMS213-2, Vienna, Austria), following the kit’s instructions. IL-6 levels were expressed as pg/ml. The intra-assay CV was 3.4%, the inter-assay CV was 5.2%, and the minimum detection limit was 0.92 pg/ml.
Analysis of TNF-α Levels
Plasma TNF-α levels were analyzed using an eBioscience ELISA kit (Catalog No: BMS223-4, Vienna, Austria). Before analysis, all reagents and samples were brought to room temperature. TNF-α levels were expressed as pg/ml. For this kit, the intra-assay CV was 6.0%, the inter- assay CV was 7.4%, and the minimum detection limit was 2.3 pg/ml.
Statistical Analysis
The data obtained from the study were analyzed using SPSS 16.0 for Windows (Chicago, IL, USA). Comparisons were made between the elite cyclist group (Group 1) and the young cyclist group (Group 2), as well as within the groups themselves. Data were presented as mean ± standard deviation. The normality of the data was tested using the Shapiro-Wilk test. The Mann-Whitney U test was used for comparisons between groups and repeated-measures ANOVA was used for within- group comparisons. p<0.05 was considered statistically significant.
Ethical Approval
This study was approved by the Ethics Committee of Non-Interventional Clinical Trials (Date: 2017-03-08, No: 2017/05).
Results
Demographic characteristics of the groups: the age of the 2nd group is lower than the 1st group (p<0.05). There was no difference in height, weight, and BMI (p>0.05).
The PTX-3 values and their time-dependent changes for the groups are presented in Table 1 and Figure 1. In Group 1, the PTX-3 value at 3 hours post-race was lower than at 1-hour post-race (p<0.05). There was no difference in PTX-3 levels between the groups (p>0.05).
The GDF-15 values and their time-dependent changes for the groups are presented in Table 2 and Figure 2. In Group 1, GDF-15 levels at 1 and 3 hours post-race were higher than pre-race and immediately post-race levels, while the GDF-15 level at 3 hours post-race was lower than at 1-hour post-race (p<0.05). In Group 2, GDF-15 levels immediately after and at 1 and 3 hours post-race were higher than pre-race levels (p<0.05). There was no difference in GDF-15 levels between the groups (p>0.05).
The IL-6 values and their time-dependent changes for the groups are presented in Table 3 and Figure 3. In Group 1, IL-6 levels immediately post-race and 1-hour post-race were higher than pre-race levels (p<0.05). The IL-6 level at 3 hours post-race was lower than immediately post-race levels (p<0.05). In Group 2, IL-6 levels were higher immediately after and 1 and 3 hours post-race, compared to pre-race levels (p<0.05). There was no difference in IL-6 levels between the groups (p>0.05). There were no differences in TNF-α levels in terms of time or between the groups (p>0.05).
Discussion
Demographic characteristics of the groups: the age of the 2nd group is lower than the 1st group (p<0.05). There was no difference in height, weight, and BMI (p>0.05).
The PTX-3 values and their time-dependent changes for the groups are presented in Table 1 and Figure 1. In Group 1, the PTX-3 value at 3 hours post-race was lower than at 1-hour post-race (p<0.05). There was no difference in PTX-3 levels between the groups (p>0.05).
The GDF-15 values and their time-dependent changes for the groups are presented in Table 2 and Figure 2. In Group 1, GDF-15 levels at 1 and 3 hours post-race were higher than pre-race and immediately post-race levels, while the GDF-15 level at 3 hours post-race was lower than at 1-hour post-race (p<0.05). In Group 2, GDF-15 levels immediately after and at 1 and 3 hours post-race were higher than pre-race levels (p<0.05). There was no difference in GDF-15 levels between the groups (p>0.05).
The IL-6 values and their time-dependent changes for the groups are presented in Table 3 and Figure 3. In Group 1, IL-6 levels immediately post-race and 1-hour post-race were higher than pre-race levels (p<0.05). The IL-6 level at 3 hours post-race was lower than immediately post-race levels (p<0.05). In Group 2, IL-6 levels were higher immediately after and 1 and 3 hours post-race, compared to pre-race levels (p<0.05). There was no difference in IL-6 levels between the groups (p>0.05).
There were no differences in TNF-α levels in terms of time or between the groups (p>0.05).
Figures
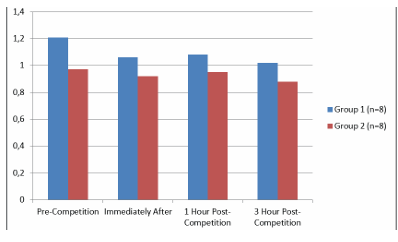
Figure 1. PTX-3 Levels of the Groups and Time-Dependent Changes
PTX- 3: Pentraxin-3. All data are presented as mean ±SD. C p<0.05 compared to 1 hour post-competition
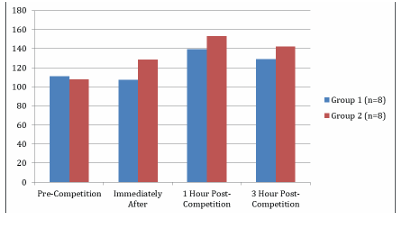
Figure 2. GDF-15 Levels of the Groups and Time-Dependent Changes
GDF-15: Growth Differentiation Factor-15. All data are presented as mean ±SD. a p<0.05 compared to pre-competition, b p<0.05 compared to 1-hour post-competition, and c p<0.05 compared to 3 hours post- competition
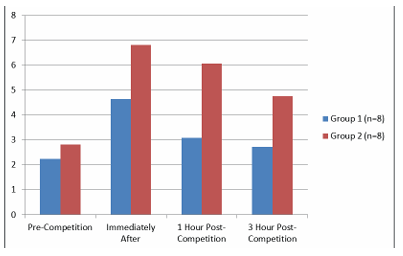
Figure 3. IL-6 Levels of theGroupsand Time-Dependent Changes
IL-6: Interleukin-6. All data are presented as mean ±SD. a p<0.05 compared to pre-competition, b p<0.05 compared to immediately after competition
Tables
Table 1. PTX-3 levels of the groups and time-dependent changes
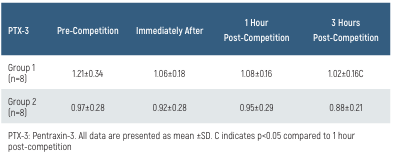
PTX-3: Pentraxin-3. All data are presented as mean ±SD. C indicates p<0.05 compared to 1 hour post-competition
Table 2. GDF-15 levels of the groups and time-dependent changes
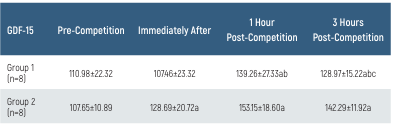
GDF-15: Growth Differentiation Factor-15. All data are presented as mean ±SD. a p<0.05 compared to pre-competition, b p<0.05 compared to 1-hour post-competition, and c p<0.05 compared to 3 hours post-competition
Table 3. IL-6 levels of the groups and time-dependent changes
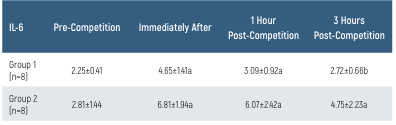
IL-6: Interleukin-6. All data are presented as mean ±SD. a p<0.05 compared to pre-competition, b p<0.05 compared to immediately after competition
Data Availability
The data supporting the findings of this article are available from the corresponding author upon reasonable request, due to privacy and ethical restrictions. The corresponding author has committed to share the de-identified data with qualified researchers after confirmation of the necessary ethical or institutional approvals. Requests for data access should be directed to bmp.eqco@gmail.com
References
-
Ye X, Wang Z, Lei W, Shen M, Tang J, Xu X, et al. Pentraxin3: A promising therapeutic target for cardiovascular diseases. Ageing Res Rev. 2024;93:102163.
-
Zlibut A, Bocsan IC, Agoston-Coldea L. Pentraxin-3 and endothelial dysfunction. Adv Clın Chem. 2019;91:163-79.
-
Ost M, Igual GC, Coleman V, Keipert S, Efstathiou S, Vidic Vandothers. Muscle-derived GDF15 drives diurnal anorexia and systemic metabolic remodeling during mitochondrial stress. EMBO reports. 2020;21(3):E48804.
-
Gallo G, Mateo-Mart M, Gotti D, Maunder E, Codella R, Ruggeri P, et al. TheWeeklyPeriodization of Top 5 Tour de France General ClassificationFinishers: A Multiple Case Study. Int J Sports Physiol Perform. 2023;18(11):1313-20.
-
Slusher AL and Huang CJ. Association of pentraxin 3 with insulin resistance and glucose response following maximal aerobic exercise in obese and normal-weight individuals. Can J PhysiolPharmacol. 2016;94(7):734–8.
-
Slusher AL, Mock JT, Whitehurst M, Maharaj A, Huang CJ. The impact of obesity on pentraxin three and inflammatory milieu to acute aerobic exercise. Metabolism. 2015;64(2):323–9.
-
Lee SE, Kang SG, Choi MJ, Jung SB, Ryu MJ, Chung HK, et al. Growth differentiation factor 15 mediates systemic glucose regulatory action of t-helper type two cytokines. Diabetes. 2017;66(11):2774-88.
-
Chung HK, Ryu D, Kim KS, Chang JY, Kim YK, Yi HS, et al. Growth differentiation factor 15 is a myomitokine governing systemic energy homeostasis. J Cell Biol. 2017;216(1):149–65.
-
Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005;54(2):114-24.
-
Pedersen BK. Exercise-induced myokines and their role in chronic diseases. Brain Behav Immun. 2011;25(5):811-6.
-
Estébanez B, Rodriguez AL, Visavadiya NP, Whitehurst M, Cuevas MJ, González-Gallego J, et al. Aerobic Training Down-Regulates Pentraxin 3 and Pentraxin 3/Toll-LikeReceptor 4 Ratio, Irrespective of Oxidative Stress Response, in Elderly Subjects. Antioxidants. 2020;9(2):110.
-
Tchou I, Margeli A, Tsironi M, Skenderi K, Barnet M, Kanaka-Gantenbein C, et al. Growth- differentiation factor-15, endoglinand N-terminal pro-brain natriuretic peptide induction in athletes participating in an ultramarathon footrace. Biomarkers. 2009;14(6):418-22.
-
Sanchis-Gomar F, Bonaguri C, Aloe R, Pareja-Galeano H, Martinez-Bello V, Gomez- Cabrera Carmen M, et al. Effects of acute exercise and xanthine oxidase inhibition on novel cardiovascular biomarkers. TranslRes. 2013;162(2):102-9.
-
Galliera E, Lombardi G, Marazzi M, Grasso D, Vianello E, Pozzoni R, et al. Acute exercise in elite rugby players increases the circulating level. Scand J Clin Lab Invest. 2014;74(6):492-9.
-
Kleinert M, Clemmensen C, Sjoberg KA, Carl CS, Jeppesen JF, Wojtaszewski JFP, et al. Exerciseincreasescirculating GDF15 in humans. Mol Metab. 2018;9:187-91.
-
Zhang H, Fealy CE, Kirwan JP. Exercise training promotes a GDF15-associated reduction in fatmass in older adults with obesity. Am J Physiol Endocrinol Metab. 2019;316(5):829-36.
-
Van Diepen JA, Berbee JF, Havekes LM, Rensen PC. Interactions between inflammation and lipid metabolism: relevance for efficacy of anti-inflammatory drugs in the treatment of atherosclerosis. Atherosclerosis. 2013;228(2):306-15.
-
Belizário JE, Fontes-Oliveira CC, Borges JP, Kashiabara JA, Vannier E. Skeletal muscle wasting and renewal: A pivotal role of myokine IL-6. Springerplus. 2016;5:619.
-
Martinez AC, Pons MM, Gomila AS, Mari JAT, Biescas AP. Changes in circulating cytokines and markers of muscle damage in elite cyclists during a multi-stagecompetition. Clin Physiol Funct Imaging. 2015;35(5):351-8.
-
Hangelbroek RWJ, Knuiman P, Tieland M, De Groot LCPGM. Attenuated strengthgains during prolonged resistance exercise trainingin older adults with high inflammatory status. Exper Ger. 2018;106:154-8.
-
Ernberg M, Christidis N, Ghafouri B, Bileviciute-Ljungar I, Löfgren M, Larsson A, et al. Effects of 15 weeks of resistance exercise on pro-inflammatory cytokine levels in the vastuslateralis muscle of patients with fibromyalgia. Arthritis Res Ther. 2016;18(1):137.
-
Windsor MT, Bailey TG, Perissiou M, Greaves K, Jha P, Leicht AS, et al. Acute Inflammatory Responsesto Exercise in Patients with Abdominal Aortic Aneurysm. Med Sci Sports Exerc. 2018;50(4):649–58.
Scientific Responsibility Statement
The authors declare that they are responsible for the article’s scientific content including study design, data collection, analysis and interpretation, writing, some of the main line, or all of the preparation and scientific review of the contents and approval of the final version of the article.
Animal and Human Rights Statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or compareable ethical standards.
Funding
None
Conflict of Interest
The authors declare that there is no conflict of interest.
Ethics Declarations
This study was approved by the Ethics Committee of Non-Interventional Clinical Trials (Date: 2017-03-08, No: 2017/05)
Additional Information
Publisher’s Note
Bayrakol MP remains neutral with regard to jurisdictional and institutional claims.
Rights and Permissions
About This Article
How to Cite This Article
Alpaslan Kisinma, Muaz Belviranli. Plasma pentraxin-3 and growth differentiation factor-15 response to acute aerobic exercise ın professional road cyclists department of physiology (medicine). Eu Clin Anal Med 2025;13(1):10-14
Publication History
- Received:
- October 4, 2024
- Accepted:
- December 17, 2024
- Published Online:
- December 30, 2024
- Printed:
- January 1, 2025
