Prognostic value of c-Kit and vascular endothelial growth factor immunoreactivity for limited-stage small-cell lung cancer
Importance of c-Kit and VEGF in lung cancer
Authors
Abstract
Aim To evaluate vascular endothelial growth factor (VEGF) and C-Kit positivity and their prognostic value in patients with limited-stage small-cell lung cancer.
Material and Methods This study utilized a retrospective cohort study design. We reviewed the demographic, clinical, and pathologic characteristics of 53 patients diagnosed with small-cell lung cancer between January 2005 and August 2010. We excluded patients with extensive disease, vena cava superior syndrome, and Eastern Cooperative Oncology Group performance status 3–4, as well as those who died in traffic accidents and those who underwent pneumonectomy. We used immunohistochemical analysis to determine vascular endothelial growth factor and C-Kit positivity in 34 patients, respectively.
Results Vascular endothelial growth factor expression was detected in 61.8% of patients, and C-Kit positivity was detected in 61.7%. Although we could not determine the prognostic significance of C-Kit positivity, vascular endothelial growth factor immunoreactivity was associated with shorter overall survival (P = 0.019; log-rank test) and poor prognosis (hazard ratio, 3.671; 95% CI, 1.257–10.723; P = 0.017) in patients with limited-stage small-cell lung cancer.
Discussion Vascular endothelial growth factor expression is an independent prognostic factor for limited-stage small-cell lung cancer.
Keywords
Introduction
Lung cancer is the leading cause of cancer-related death, both in Turkey and worldwide. Small-cell lung cancer (SCLC) is an aggressive form of lung cancer with a poor prognosis. Despite the use of new chemotherapeutic agents, little progress has been made toward prolonging the survival of SCLC patients. No targeted therapies are currently available for the treatment of SCLC 1.
Numerous studies have shown that clinicopathological characteristics, including performance status, tumor stage, age, gender, number of metastatic sites, and weight loss, have prognostic value in SCLC. In addition to these clinicopathological characteristics, serum albumin (Alb), sodium (Na), alkaline phosphatase (ALP), and lactate dehydrogenase levels (LDL) have been identified as biochemical variables with prognostic significance in SCLC. Extensive investigations are underway to identify novel and robust predictive and prognostic biomarkers in SCLC 2,3,4.
Angiogenesis is essential for cancer development and progression. Among angiogenetic factors, vascular endothelial growth factor (VEGF) has attracted increasing attention because of its critical role in pathological angiogenesis 5. Autocrine hormones, such as CD 117 (C-Kit), also play a key role in tumor growth and the progression of many cancers, including SCLC 6.
However, the prognostic value of these pro-tumorigenic molecules in SCLC remains unclear. In this study, we assessed the alteration in VEGF and C-Kit positivity in SCLC and evaluated their prognostic value for limited-stage SCLC (LS-SCLC).
Materials and Methods
Participants
We retrospectively reviewed the demographic, clinical, and pathological characteristics of patients diagnosed with SCLC between January 2005 and August 2010. The clinical and demographic factors included age, gender, smoking status, Eastern Cooperative Oncology Group (ECOG) performance status, tumor stage (according to the staging system of the Veterans Administration Lung Cancer Study Group), progression- free survival (PFS), and overall survival (OS). We also reviewed the results of renal and liver function tests and obtained data on ALP serum calcium (Ca) levels and complete blood count (CBC). Additionally, we evaluated posteroanterior and lateral chest radiographs and computed tomography (CT) scans of the thorax, as well as cranial CT, bone positron emission tomography (PET)-CT, and skeletal scintigraphy scans.
Weight loss was defined as an at least 10% loss of body weight during the last 6 months. The treatment modalities were categorized into four groups: no treatment (patients who refused chemotherapy and radiotherapy), chemotherapy (CT), neoadjuvant chemotherapy followed by radiotherapy (NACT), and concurrent chemoradiotherapy (CCRT). Out of 53 patients diagnosed with SCLC, we excluded patients with extensive disease (n = 9), vena cava superior syndrome (n = 4), ECOG performance status 3–4 (n = 4), or death due to traffic accident (n = 1), as well as those who underwent pneumonectomy (n = 5). A standard etoposide cisplatin (EP) regimen was used in all patients except for one patient who received vincristine, adriamycin, and cyclophosphamide due to an allergic reaction to the etoposide cisplatin regimen. Eight patients refused treatment. The response to treatment was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines, version 1.1 7; tumor response was classified as stable disease, partial response, complete response, or progressive disease. VEGF and C-Kit positivity levels were assessed by immunohistochemical staining of tissues from 34 patients, respectively.
Immunohistochemistry
The extent of VEGF and C-Kit expression was assessed using a semi-quantitative scoring method based on the percentage of positive cells and staining intensity, ensuring consistency and reproducibility in the analysis. SCLC specimens were collected before chemotherapy (CT) or radiotherapy (RT). Immunostaining for C-Kit (NeoMarkers, 1/100 dilution) and VEGF (NeoMarkers, 1/100) was performed using the streptavidin- biotin method with mouse monoclonal antibodies. Sections (6-µm thick) were prepared from formalin-fixed, paraffin-embedded tissue specimens and mounted on Poly-L-lysine-coated slides. Tissues were dewaxed in xylene, rehydrated, and washed in phosphate buffer (pH 7.6) for 10 min. Epitope retrieval was performed in citrate buffer for C-Kit and in EDTA for VEGF. Immunostaining was evaluated using a streptavidin-biotin detection kit (Lab Vision). After incubation with the chromogen, sections were counterstained with Harris’s hematoxylin, and the coverslipped. Skin and angiosarcoma tissues were used as positive controls for C-Kit and VEGF positivity, respectively. In negative controls, the primary antibody was omitted. The intensity of C-Kit and VEGF staining was evaluated by light microscopy 8.
Statistical analysis
We used Pearson’s chi-square test, Yates’ chi-square test, or Fisher’s exact test to compare differences between groups in categorical variables. Survival analyses were conducted using the Kaplan-Meier method. OS was determined from the day of pathological diagnosis until the time of death. PFS (in months) was calculated from the day of pathological diagnosis until the time of disease progression. The log- rank test was used to compare the survival curves generated by the univariate analysis. The prognostic value of C-Kit and VEGF for LS-SCLC was evaluated by multivariate analyses using the Cox proportional- hazards model. Age, weight loss, hypoalbuminemia, treatment modality, and C-Kit and VEGF immunoreactivity were used as independent variables in the Cox proportional-hazards model. Binary logistic regression with the backward stepwise selection method was used to identify significant independent predictors of patient survival. Variables with P < 0.250 in this analysis were included in the Cox proportional hazards model. All statistical analyses were performed using SPSS for Windows software (version 15.0; SPSS Inc.). P-values < 0.05 were considered statistically significant.
Ethical approval
This study was approved by the Ethical Board of Chest Diseases and Thoracic Surgery Research and Education Hospital, Ankara, Turkey (Date: 2022-01-12, No: 25). The study was performed in accordance with the principles stated in the Declaration of Helsinki. Written informed consent was obtained from all participants using appropriate patient consent forms.
Results
The demographics and clinical characteristics of patients are summarized in Table 1. Most SCLC patients were men with a history of smoking. Poor prognostic factors, including weight loss, hypoalbuminemia, and elevated LDH levels, were observed in approximately 25% of the patients. Hypoalbuminemia was a significant predictor of a poor prognosis (P = 0.029; log-rank test), whereas the prognostic value of an elevated LDH level and weight loss did not reach statistical insignificance in this cohort (P = 0.486 and P = 0.217, respectively; log-rank test) (Table 1.).
VEGF positivity was detected in 61.8% of the patients, and C-Kit positivity was detected in 61.7%. The median OS was 13 months (range, 1–105 months), and the median PFS was 8 months (range: 1–91 months). The median OS was 15 months longer in the VEGF (−) group than in the VEGF (+/++) group; this survival advantage was statistically significant (P = 0.019; log-rank test). The median PFS of patients in the VEGF (−) group was 13 months, whereas that of patients in the VEGF (+) group was 7 months (P > 0.050; log-rank test). We found no significant difference in the median OS or PFS between patients in the c-Kit (−) and C-Kit (+) groups (P > 0.050, log-rank test; Figures 1 and 2). We also investigated the relationship between C-Kit and VEGF immunoreactivities and the response to treatment. We found no significant association between the response to standard therapy and C-Kit or VEGF positivity (P = 0.305 and P = 0.289, respectively). Additionally, treatment modality was not significantly associated with patient survival (P = 0.248); however, patients treated with CCRT or NACT survived longer than patients treated with chemotherapy alone and non-treated patients (Table 2.). Multivariate analysis (Cox regression) was performed to determine the prognostic significance of age, weight loss, hypoalbuminemia (serum Alb < 3.5 g/ dL), treatment modality, and C-Kit and VEGF immunoreactivity (Table 3.). Only VEGF immunoreactivity was a significant independent predictor of a poor prognosis (hazard ratio [HR], 3.671; 95% confidence interval [CI], 1.257–10.723; P = 0.017). C-Kit was localized in the cell membrane. C-Kit positivity was defined as negative (−), 10% positive cells (+), 10%–50% positive cells (++), and >50% positive cells (+++) (Figure 3A-B.). VEGF was localized in the cytoplasm. The extent of expression was graded as focal (up to 50% of cells) or diffuse (> 50% of cells) (Figure 3C-D.).
Discussion
Even though several novel targeted therapies and immunotherapies have shown promising antitumor effects in patients with lung cancer, the treatment of SCLC remains challenging despite its chemosensitivity, and the survival outcomes of SCLC patients remain unsatisfactory 1,9,10. Angiogenesis is essential for tumor growth and metastasis, and VEGF is a key pro-angiogenic factor 11,12. Studies have shown that SCLC tumors were characterized by high intratumoral microvascular density, which was strongly correlated with VEGF positivity levels 15. The autocrine growth factor C-Kit has also been implicated in many types of cancer, including SCLC 13,14,15. Nevertheless, the prognostic roles of VEGF and C-Kit in SCLC remain understudied.
In this study, we investigated the relationships of C-Kit and VEGF immunoreactivities with LS-SCLC prognosis. Previous studies have shown that VEGF immunoreactivity in SCLC ranged widely (between 31% and 81%), possibly due to the use of different methods to evaluate VEGF positivity 15. Here, we show that VEGF was expressed in 61.8% of SCLC patients. The reported expression of C-Kit also varies widely among studies, from 28% to 88% 16,17,18. In this study, we detected C-Kit positivity in 61.7% of our patients. We also found that c-Kit immunoreactivity had no effect on the OS, PFS, or treatment response of patients with LS-SCLC, in line with the results of some previous studies 17,18. However, other studies have shown an association between C-Kit positivity and SCLC patient survival 16,17,18. Several factors may have led to these conflicting results, including differences in tissue specimens (paraffinized blocks, tissue cultures, or fresh- frozen sections), immunohistochemical criteria, and patient selection criteria (e.g., presence of risk factors, comorbidities, and disease stage). Our results suggest that C-Kit positivity alone cannot predict survival outcomes in LS-SCLC patients with ECOG 1–2 and no venous disease.
Smoking cessation, younger age, gender, elevated LDH serum levels, CCRT, and platinum-based chemotherapy have been identified as prognostic factors in LS-SCLC (5, 25). Nonetheless, there are conflicting results regarding the prognostic value of VEGF positivity in SCLC 19. A meta-analysis of five studies revealed a significant association between VEGF positivity and SCLC patient survival (HR, 1.41; 95% CI, 1.17– 1.65; P = 0.04) (17). In this study, we investigated the prognostic potential of VEGF positivity in a more homogenous cohort of LS-SCLC patients and found that VEGF positivity was associated with a significantly poorer OS. However, the association between VEGF positivity and PFS was not statistically significant. Thus, the relationship between VEGF positivity and PFS in LS-SCLC patients warrants further investigation in large-cohort prospective studies.
Puri et al. demonstrated that VEGF mRNA levels were positively correlated with C-Kit positivity levels 20. In this study, we identified VEGF immunoreactivity as an independent risk factor for LS-SCLC. However, we found no association between VEGF immunoreactivity and the response to standard treatment, highlighting the need for novel therapies to treat VEGF (+) LS-SCLC. Emerging therapies against VEGF (+) LS-SCLC include the anti-VEGF antibody bevacizumab. However, despite promising preclinical findings, clinical trials are required to confirm the usefulness of bevacizumab in patients with LS-SCLC 22.
VEGF’s identification as an independent risk factor underscores its potential as a key biomarker in guiding treatment strategies for LS- SCLC. While the current findings are preliminary, they highlight the need to explore VEGF-targeted therapies, such as bevacizumab, which could improve survival outcomes in patients exhibiting VEGF overexpression. Integrating VEGF inhibitors into treatment regimens may offer a personalized approach to managing LS-SCLC, potentially enhancing the efficacy of conventional chemotherapy or chemoradiotherapy.
Figures

Figure 1. Associations between VEGF immunoreactivity and (A) progression-free survival (B) and overall survival

Figure 2. Associations between c-Kit immunoreactivity and (A) progression-free survival and (B) overall survival

Figure 3. Representative images showing (A) c-Kit–positive membranous immunostaining and (B) c-Kit–negative cytoplasmic immunostaining in specimens obtained from patients with small-cell lung cancer (hematoxylin-eosin; magnification ×400 magnification). Representative images showing (C) strong VEGF-positive immunostaining and (D) VEGF- negative immunostaining in specimens obtained from patients with small-cell lung cancer (hematoxylin-eosin; magnification ×400). VEGF, vascular endothelial growth factor
Tables
Table 1. Demographic and clinical characteristics of the study cohort
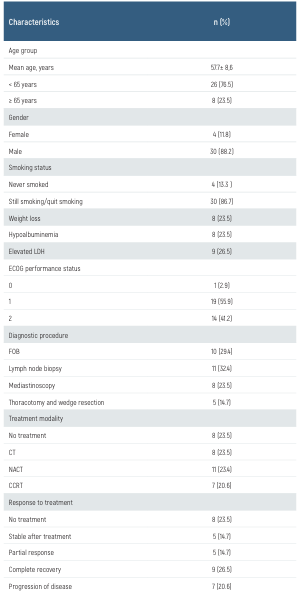
CCRT: concurrent chemoradiotherapy, CT: chemotherapy, ECOG: Eastern Cooperative Oncology Group, FOB: fiber optic bronchoscopy, LDH: lactate dehydrogenase, NACT: neoadjuvant chemotherapy followed by radiotherapy
Table 2. Overall survival by treatment modality
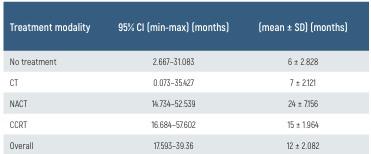
CCRT: concurrent chemoradiotherapy, CT: chemotherapy, NACT: neoadjuvant chemotherapy followed by radiotherapy
Table 3. Multivariate analysis of prognostic factors in LS-SCLC patients
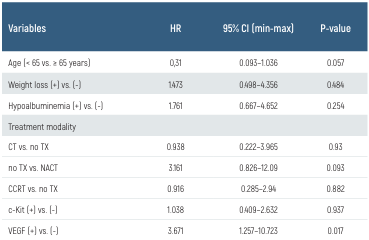
CCRT: concurrent chemoradiotherapy, c-Kit: CD117, CT: chemotherapy, HR: hazard ratio, TX: transthoracic biopsy, VGEF: vascular endothelial growth factor
Limitations
This study has certain limitations. The cross-sectional design of this study presents inherent limitations, particularly the inability to establish causal relationships. Additionally, potential confounding factors such as smoking history, prior treatments, and comorbidities could influence VEGF positivity and overall survival outcomes. Although the study controlled for some variables, unmeasured factors might still contribute to the observed associations. Future longitudinal studies with larger, more diverse cohorts are essential to validate these findings and refine the role of VEGF in therapeutic decision-making for SCLC.
Conclusion
Our findings suggest that VEGF immunoreactivity can be used as a poor prognostic factor in LC-SCLC. Further comprehensive studies are required to elucidate the prognostic and therapeutic value of VEGF and C-Kit positivity in LC-SCLC.
Data Availability
The data supporting the findings of this article are available from the corresponding author upon reasonable request, due to privacy and ethical restrictions. The corresponding author has committed to share the de-identified data with qualified researchers after confirmation of the necessary ethical or institutional approvals. Requests for data access should be directed to bmp.eqco@gmail.com
References
-
Hernandez-Martinez JM, Guijosa A, Flores-Estrada D, Cruz-Rico G, Turcott J, Hernandez- Pedro N, et al. Real-World Survival Outcomes in Non-Small Cell Lung Cancer: The Impact of Genomic Testing and Targeted Therapies in a Latin American Middle-Income Country. JCO Glob Oncol. 2024;10(12):1-10.
-
An L, Yin Wt, Sun DW. Albumin-to-alkaline phosphatase ratio as a promising indicator of prognosis in human cancers: is it possible? BMC Cancer. 2021;21(1):1-7.
-
Liang H, Ma D, Xu Y, Zhao J, Chen M, Liu X, et al. Elevated levels of pre-treatment lactate dehydrogenase are an unfavorable predictor factor in patients with EML4-ALK rearrangement non-small cell lung cancer treated with crizotinib. Cancer Manag Res. 2019;11(1):8191-200.
-
Simic I, Guzonjic A, Kotur Stevuljevic J, Ceriman Krstic V, Samardzic N, Savic Vujovic K, et al. Correlation of Systemic Inflammation Parameters and Serum SLFN11 in Small Cell Lung Cancer—A Prospective Pilot Study. Biomedicines. 2024;12(5):1-12.
-
Zeng J, Deng Q, Chen Z, Yan S, Dong Q, Zhang Y, et al. Recent development of VEGFR small molecule inhibitors as anticancer agents: A patent review (2021–2023). Bioorganic Chemistry. 2024;146(1):1-14.
-
Kim KH, Kim JO, Park JY, Seo MD, Park SG. Antibody-Drug Conjugate Targeting c-Kit for the Treatment of Small Cell Lung Cancer. Int J Mol Sci. 2022;23(4):2264.
-
Jang GS, Kim MJ, Ha HI, Kim JH, Kim HS, Ju SB, et al. Comparison of RECIST version 1.0 and 1.1 in assessment of tumor response by computed tomography in advanced gastric cancer. Chin J Cancer Res. 2013;25(6):689-94.
-
Donati V, Faviana P, Dell’omodarme M, Prati MC, Camacci T, De Ieso K, et al. Applications of tissue microarray technology in immunohistochemistry: a study on c-kit expression in small cell lung cancer. Hum Pathol. 2004;35(11):1347-52.
-
Hu D, Zhou YY, Ma HB, Tao MM, Huang QZ, Yang ZZ, et al. Efficacy and safety of EGFR- TKIs in combination with angiogenesis inhibitors as first-line therapy for advanced EGFR- mutant non-small-cell lung cancer: a systematic review and meta-analysis. BMC Pulm Med. 2023;23(1):207-17.
-
Cheema PK, Wheatley-Price PF, Cecchini MJ, Ellis PM, Louie AV, Moore S, et al. Update on Practical Management of Early-Stage Non-Small Cell Lung Cancer (NSCLC): A Report from the Ontario Forum. Curr Oncol. 2024;31(11):6979-99.
-
Zhao W, Jiang J. Advances in Predictive Biomarkers for Anti-Angiogenic Therapy in Non- Small Cell Lung Cancer. Cancer Control. 2024;31(12):1-13.
-
He C. Activating Invasion and Metastasis in Small Cell Lung Cancer: Role of the Tumour Immune Microenvironment and Mechanisms of Vasculogenesis, Epithelial-Mesenchymal Transition, Cell Migration, and Organ Tropism. Cancer Rep (Hoboken). 2024;7(10):1-11.
-
Alharthi NS, Al-Zahrani MH, Hazazi A, Alhuthali HM, Gharib AF, Alzahrani S, et al. Exploring the lncRNA-VEGF axis: Implications for cancer detection and therapy. Pathol Res Pract. 2024;253(11):1-9.
-
Li MSC, Mok KKS, Mok TSK. Developments in targeted therapy & immunotherapy-how non-small cell lung cancer management will change in the next decade: A narrative review. Ann Transl Med. 2023;11(10):358-67.
-
Wang Q, Zeng A, Zhu M, Song L. Dual inhibition of EGFR-VEGF: An effective approach to the treatment of advanced non-small cell lung cancer with EGFR mutation (Review). Int J Oncol. 2023;62(2):1-8.
-
Kim KH, Kim JO, Park JY, Seo MD, Park SG. Antibody-Drug Conjugate Targeting c-Kit for the Treatment of Small Cell Lung Cancer. Int J Mol Sci. 2022;23(4):1-9.
-
Su Y, Chen R, Han Z, Xu R, Ma L, Wufuli R, et al. Clinical and Prognostic Significance of CD117 in Non-Small Cell Lung Cancer: A Systemic Meta-Analysis. Pathobiology. 2021;88(4):267-76.
-
Zhou Y, Wang L, Sun Z, Zhang J, Wang X. Targeting c-kit inhibits gefitinib resistant NSCLC cell growth and invasion through attenuations of stemness, EMT and acquired resistance. Am J Cancer Res. 2020;10(12):4251-65.
-
Rosique-Aznar C, Valcuende-Rosique A, Rosique-Robles D, Sanchez-Alcaraz A. Relationship between Lactate Dehydrogenase and survival in patients with non-small cell lung cancer receiving immunotherapy. Farm Hosp. 2024;10(1):1-8.
-
Puri S, Kaur G, Piplani H, Sanyal SN, Vaish V. Imatinib modulates pro-inflammatory microenvironment with angiostatic effects in experimental lung carcinogenesis. Inflammopharmacology. 2020;28(1):231-52.
-
Jeong H, Kim RI, Koo H, Choi YH, Kim M, Roh H, et al. Stem cell factor and cKIT modulate endothelial glycolysis in hypoxia. Cardiovasc Res. 2024;120(7):745-55.
-
Rinaldi I, Mauludi R, Jusman SW, Sinto R, Harimurti K. HIF2-alpha Expression in CML Patients Receiving Hydroxyurea Prior to Imatinib That Achieved Major Molecular Response (MMR) versus in Those Not Achieving MMR. J Blood Med. 2024;15(2):61-7.
Scientific Responsibility Statement
The authors declare that they are responsible for the article’s scientific content including study design, data collection, analysis and interpretation, writing, some of the main line, or all of the preparation and scientific review of the contents and approval of the final version of the article.
Animal and Human Rights Statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or compareable ethical standards.
Funding
None
Conflict of Interest
The authors declare that there is no conflict of interest.
Ethics Declarations
This study was approved by the Ethics Committee of Board of Chest Diseases and Thoracic Surgery Research and Education Hospital, Ankara (Date: 2022-01-12, No: 25)
Additional Information
Publisher’s Note
Bayrakol MP remains neutral with regard to jurisdictional and institutional claims.
Rights and Permissions
About This Article
How to Cite This Article
Gulen Ece Topaloglu, Sezgi Sahin Duyar, Aydin Yilmaz, Funda Demirag, Yurdanur Erdogan, Merter Bora Erdogdu, Ulku Yilmaz. Prognostic value of C-Kit and vascular endothelial growth factor immunoreactivity for limited-stage small-cell lung cancer. Eu Clin Anal Med 2025;13(1):1-5
Publication History
- Received:
- December 7, 2024
- Accepted:
- December 31, 2024
- Published Online:
- December 31, 2024
- Printed:
- January 1, 2025
